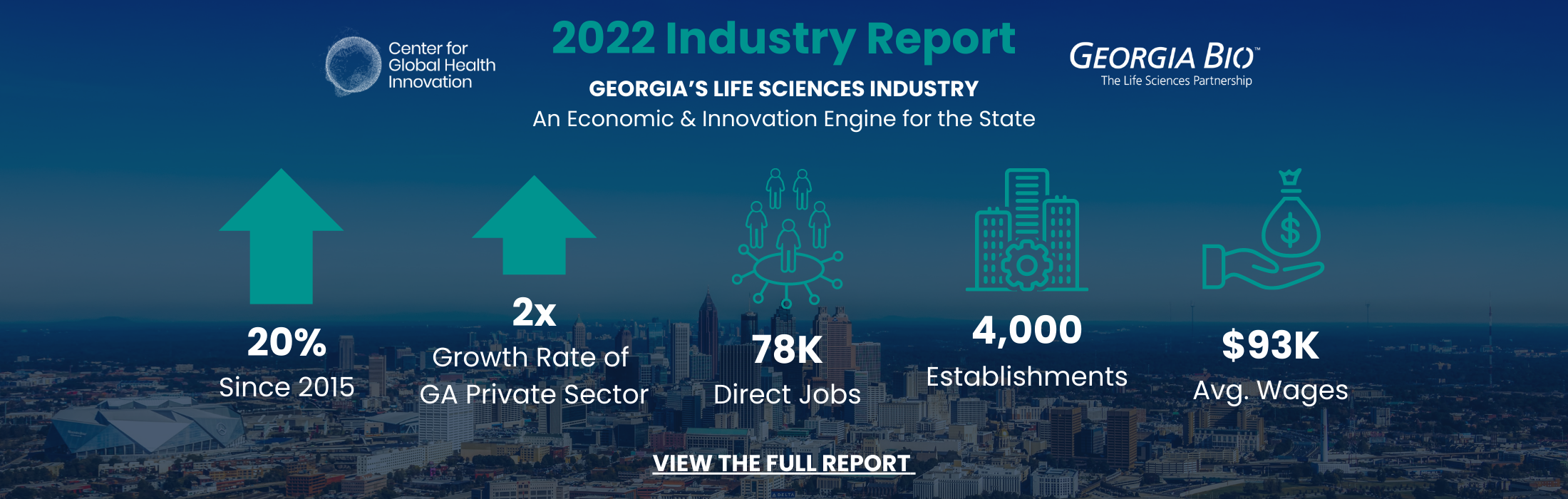
0
0
0
0
Georgia Bio is an independent, non-profit 501(c)(6) trade association whose mission is to advance the growth of Georgia’s life sciences industry and foster strategic partnerships that can create a healthier world. To accomplish this mission, Georgia Bio works on behalf of 200 member organizations to drive public policy, build a network of industry leaders, create access to capital, introduce cutting-edge STEM education programs, and create robust value-driven purchasing programs.
Summer 2023, Georgia Bio recommitted to its core mission of advocating for, connecting, educating and inspiring our member enterprises and stakeholders in much the same way our colleagues have known Georgia Bio since its inception in 1989. To achieve this, the organization transitioned from a 501c3 to an independent 501c6 organization, Georgia Biosciences Organization, Inc. You can read more about this on our blog here. Our new EIN# is 27-3855537.
Chairman's Circle
Champion Members
View Latest News
Exciting news for Georgia Bio members! The Georgia Bio Brex program, established through our partnership with BIO, has been expanded…
Read MoreMarch 13th, join a conversation with Radically Rural biotech: Why and how rural biotech hubs can compete with urban hubs.…
Read MoreArtelon®, a leader in dynamic biomaterial innovation, is expanding its Manufacturing and Research and Development activities to BioSpark Labs. “This…
Read More